A Multi-Omics Approach to Gastric Cancer Classification: Identifying Distinct Molecular Subtypes for Enhanced Therapeutic Outcomes
Code: G-1511
Authors: Setayesh Zarei ℗, Fatemeh Dorri, Modjtaba Emadi-Baygi *
Schedule: Not Scheduled!
Tag: Cancer Diagnosis & Treatment
Download: Download Poster
Abstract:
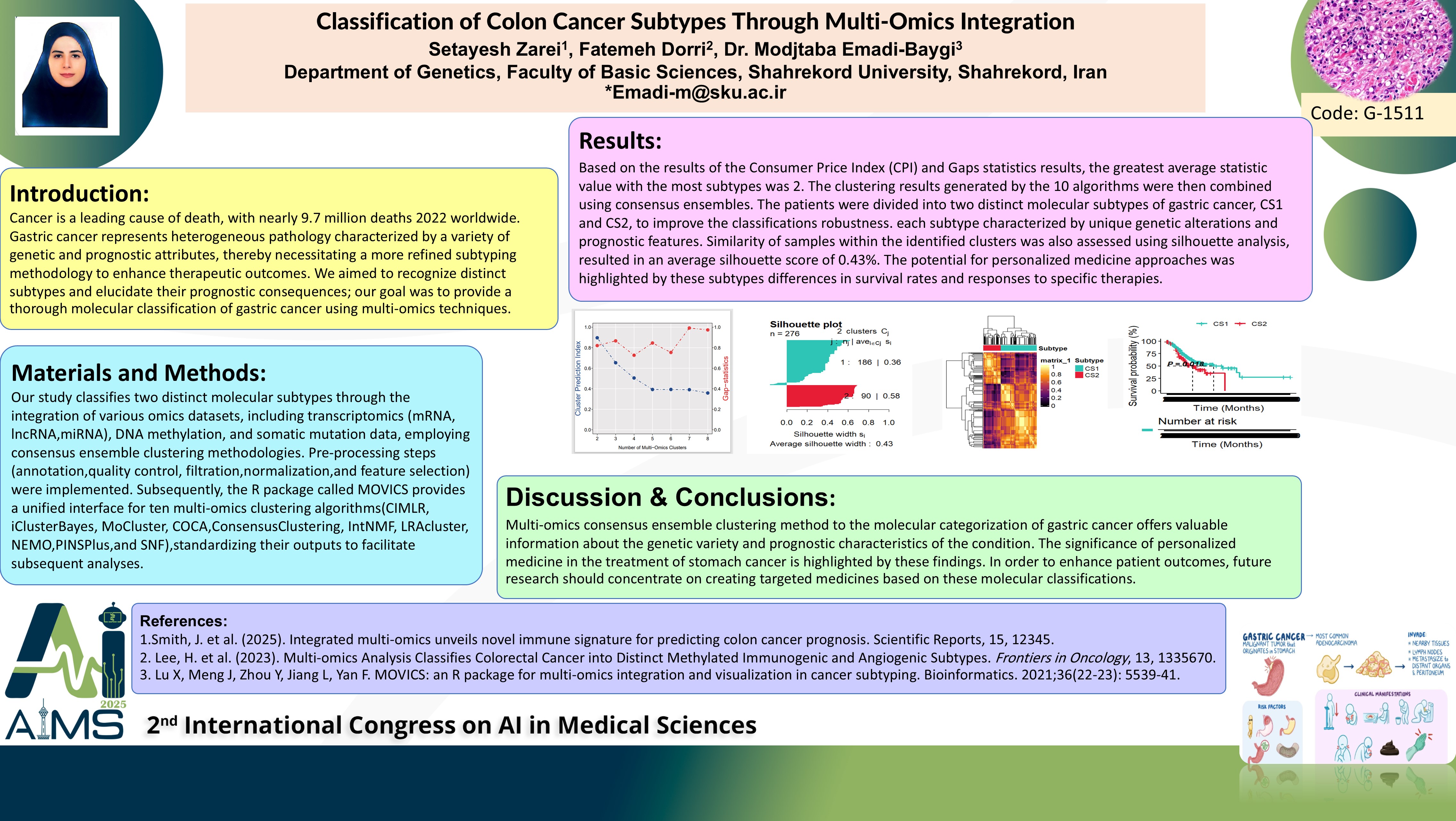
Abstract
Background and aims: Cancer is a leading cause of death. It represents a heterogeneous pathology characterized by a variety of genetic and prognostic attributes, necessitating a more refined subtyping methodology to enhance therapeutic outcomes. Here, we aimed to identify distinct molecular subtypes of gastric cancer through multi-omics integration, elucidating their prognostic implications and potential applications in personalized medicine. Method: To achieve robust classification, we integrated multiple omics datasets employing consensus ensemble clustering methodologies. Pre-processing steps (annotation, quality control, filtration, normalization, and feature selection) were implemented. Subsequently, the R package called MOVICS provided a unified interface for ten multi-omics clustering algorithms (CIMLR, iClusterBayes, MoCluster, COCA, ConsensusClustering, IntNMF, LRAcluster, NEMO, PINSPlus, and SNF), standardizing their outputs to facilitate subsequent analyses. The optimal number of clusters was determined using two widely accepted metrics: the Calinski-Harabasz Index and Gap statistics. Results: Based on the CHI and Gap statistics, the highest average statistic value corresponded to two distinct molecular subtypes of gastric cancer: CS1 and CS2. The clustering results generated by the 10 algorithms were then combined using consensus ensembles. The patients were divided into two distinct molecular subtypes: CS1 and CS2, to improve the classification's robustness. Each subtype was characterized by unique genetic alterations and prognostic features. Similarity of samples within the identified clusters was assessed using silhouette analysis, resulting an average silhouette score of 0.43. The potential for personalized medicine approaches was highlighted by these subtypes' differences in survival rates and responses to specific therapies. Conclusion: The application of multi-omics consensus ensemble clustering to the molecular categorization of gastric cancer offers valuable information about the genetic diversity and prognostic characteristics of the condition. These findings highlight the significance of personalized medicine in the treatment of stomach cancer. Future research should focus on developing targeted therapies based on these molecular classifications to enhance patient outcomes.
Keywords
Multi-Omics, Cancer Subtyping, Prognostic Features